Calcium (Ca) is an abundant element in the Earth’s crust. The most abundant forms of calcium are calcium carbonate (CaCO3), the main constituent of marble and travertine and calcium sulfate (CaSO4), the main component of plaster. Calcium in water supplies comes from water passing over deposits of limestone, dolomite, gypsum, and gypsiferous shale. The concentration may extend from zero to several hundred milligrams per liter, depending on the source and treatment.
Calcium in water as carbonate is a primary component of water hardness that can cause pipe or tube scaling. Classifying the degree of hardness of water is a meausrement of the parameter “total hadness” which is the total sum of the Ca2+ and Mg2+ ions.
A main use of calcium is in the production of cements and mortars for the construction of buildings. It is also used as a deoxidizing, desulphurizing, and decarburizing agent for various ferrous alloys, as well as a binding agent in the production of aluminum, lead, magnesium, and beryllium alloys. The Ca2+ ion plays a crucial role in cell physiology and biochemistry. As a building block of animal bones and shells, calcium is essential for animal nutrition. Calcium also helps plant roots develop and increases the resistance and strength of plant tissues and stems
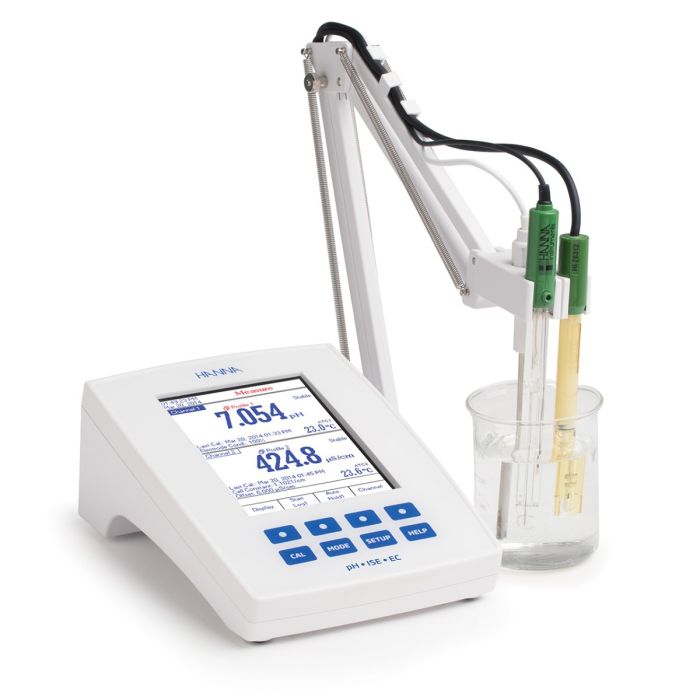
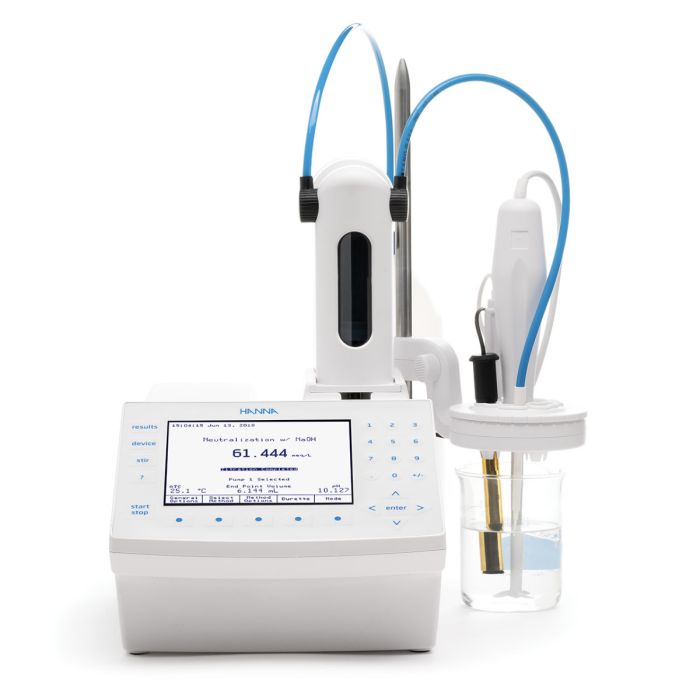
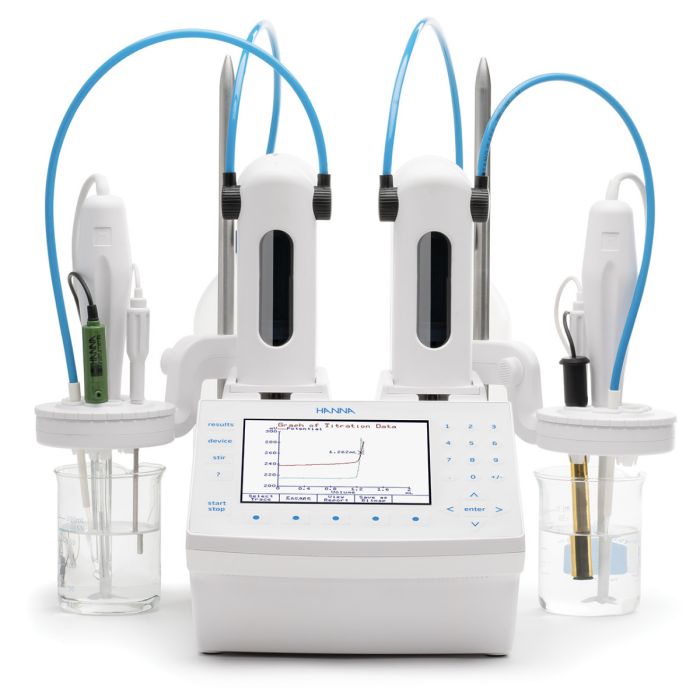
Hanna Instruments offers a variety of methods to measure calcium. Products include portable and benchtop pH/ISE meters that can use a liquid membrane calcium Ion-Selective Electrode (ISE) for direct measurement. Titrations for calcium can be performed with the calcium ISE or a photometric probe using EDTA as a titrant.
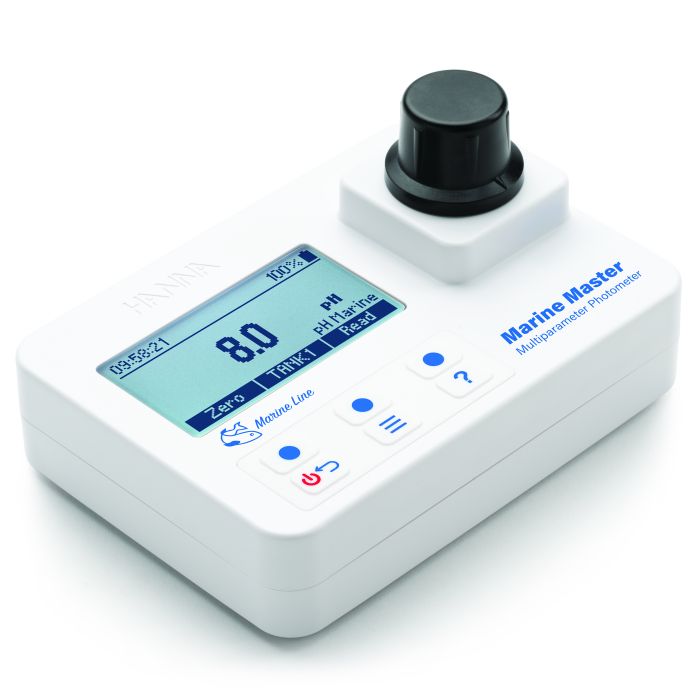
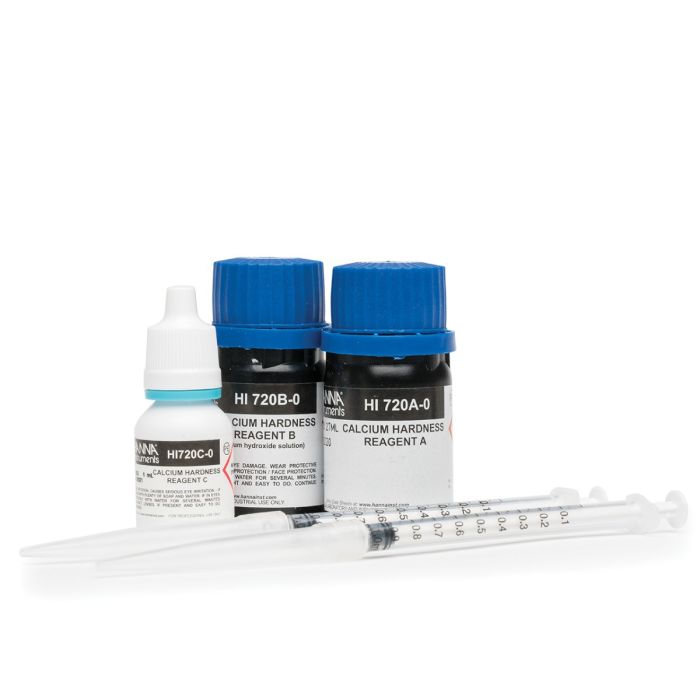
Calcium is also measured photometrically with reagents. Photometric analysis is based on the Beer-Lambert principle of absorbance. Photometric analysis products include handheld colorimeters, portable and benchtop photometers, and spectrophotometers. Photometric methods include reagent chemistries based on the Calmagite method found in the Standard Methods for the Examination of Water and Wastewater, Oxalate method, and the Zincon method used for measuring calcium in the saltwater aquarium for reef maintenance.
Calcium is commonly expressed as mg/L of calcium carbonate (CaCO3). The portable and benchtop photometers allow calcium to be expressed as French degrees (of), German degrees (odH) and English degrees (oE).